
Gibbs Free Energy Change & Gibbs Equation (5.2.3) | CIE A Level Chemistry Revision Notes 2022 | Save My Exams

thermodynamics - Reaction quotient and Gibbs free energy at the start of a reaction - Chemistry Stack Exchange
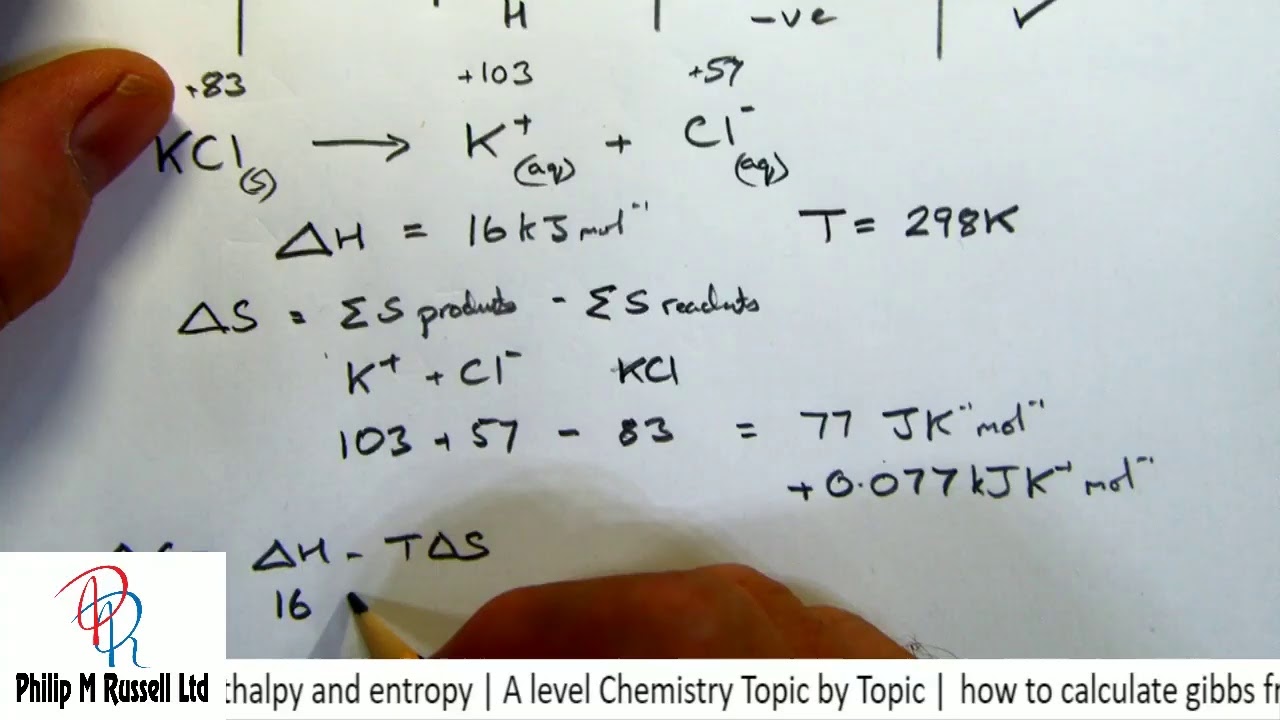
how to calculate gibbs free energy from enthalpy and entropy | A level Chemistry Topic by Topic - YouTube
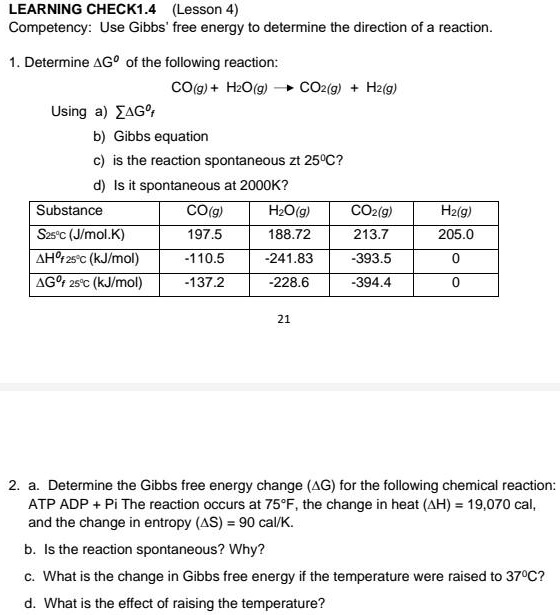
SOLVED: LEARNING CHECK1.4 (Lesson 4) Competency: Use Gibbs' free energy t0 determine the direction of a reaction. Determine AG" of the following reaction: CO(g) + HzO(g) COz(g) Hz(g) Using a) ZAG% b)

Chemical Energetics: Application of Gibbs Free Energy in Thermodynamics - A-Level H2 Chemistry Tuition by 10 Year Series Author

How to Find Standard Gibbs Free Energy of Reaction from Standard Gibbs Free Energies of Formation | Chemistry | Study.com
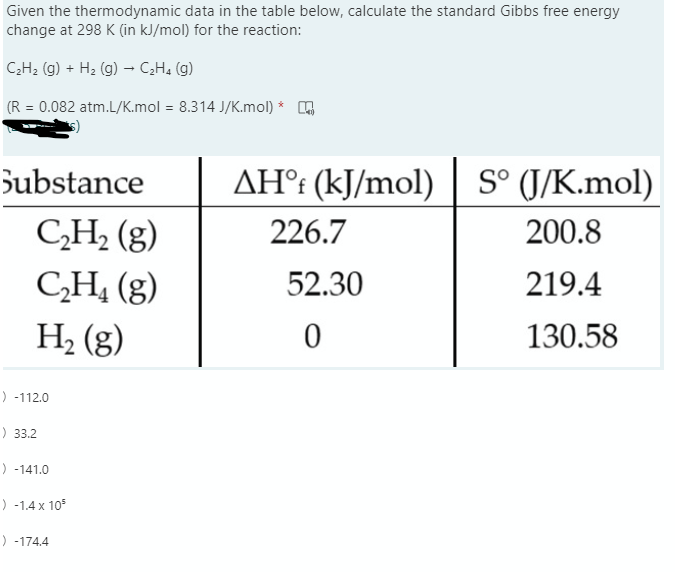
![Calculate the standard Gibbs free energy change from the free energies of formation data for the following reaction: C(6)H(6)(l) +(15)/(2)O(2)(g) rarr 6CO(2)(g) +3H(2)O(g) Given that Delta(f)G^(Theta) =[C(6)H(6)(l)] = 172.8 kJ mol^(-1) Delta(f)G^(Theta ... Calculate the standard Gibbs free energy change from the free energies of formation data for the following reaction: C(6)H(6)(l) +(15)/(2)O(2)(g) rarr 6CO(2)(g) +3H(2)O(g) Given that Delta(f)G^(Theta) =[C(6)H(6)(l)] = 172.8 kJ mol^(-1) Delta(f)G^(Theta ...](https://d10lpgp6xz60nq.cloudfront.net/web-thumb/11035720_web.png)
Calculate the standard Gibbs free energy change from the free energies of formation data for the following reaction: C(6)H(6)(l) +(15)/(2)O(2)(g) rarr 6CO(2)(g) +3H(2)O(g) Given that Delta(f)G^(Theta) =[C(6)H(6)(l)] = 172.8 kJ mol^(-1) Delta(f)G^(Theta ...




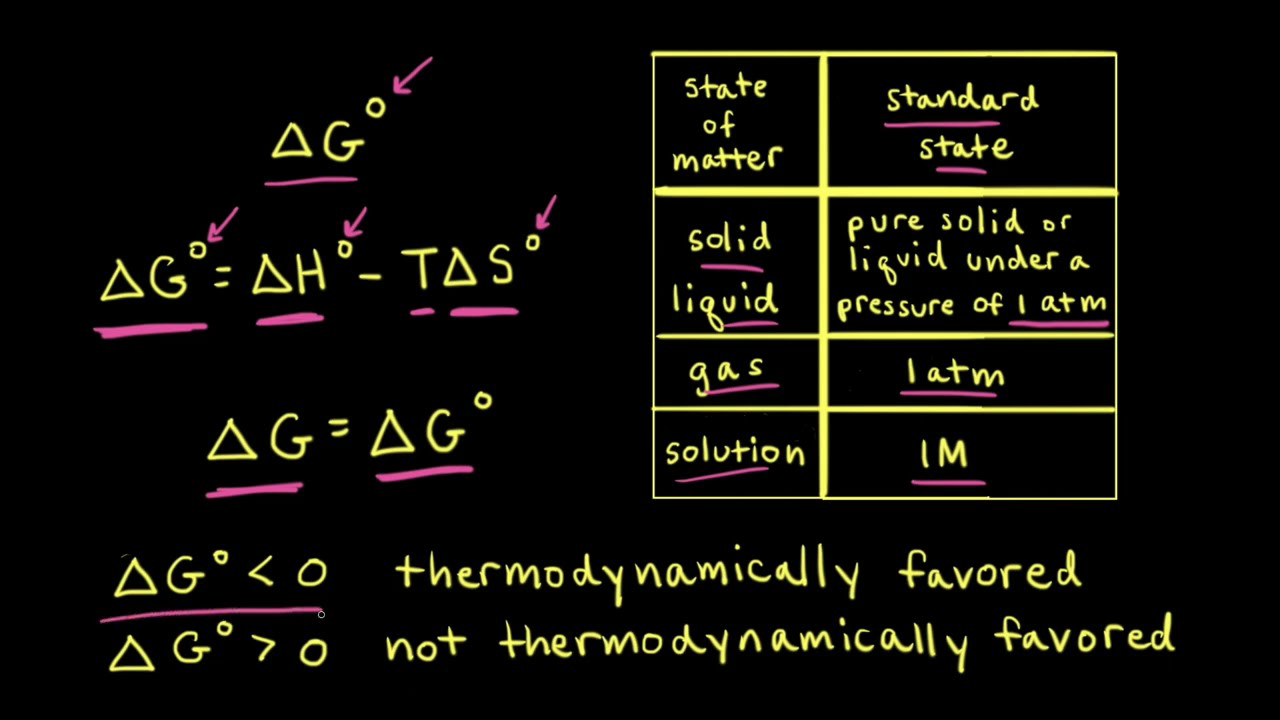



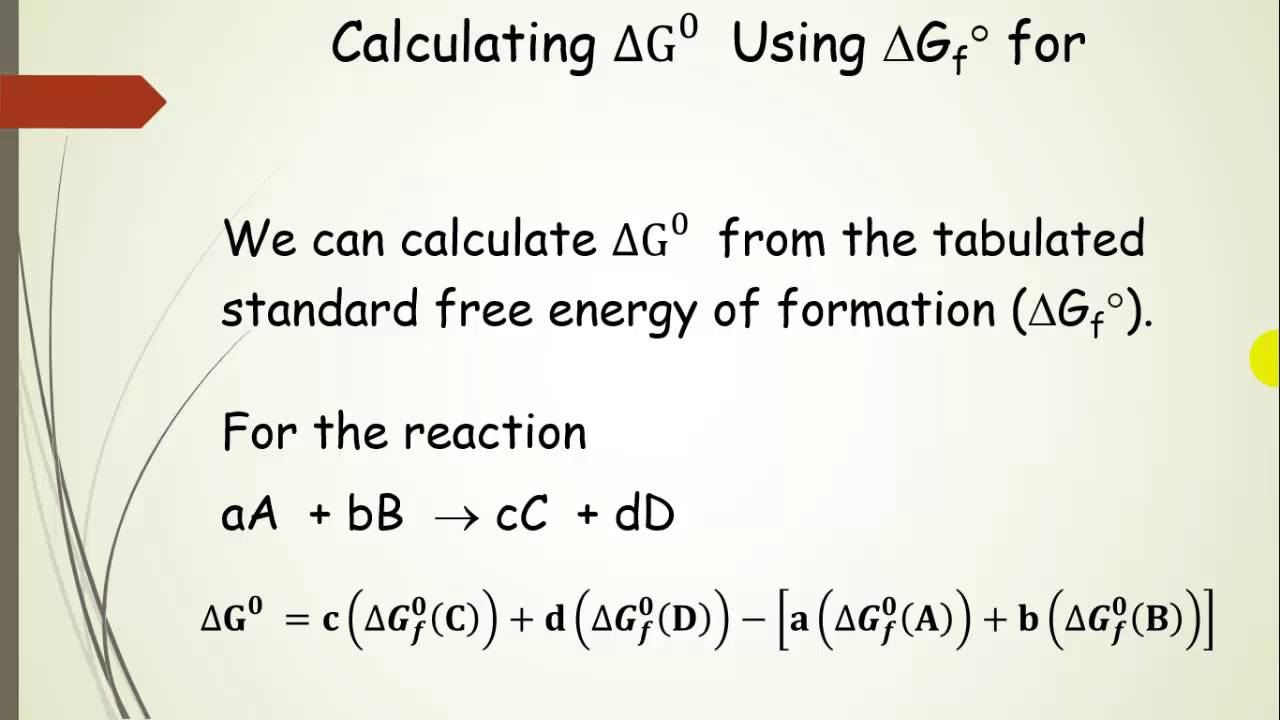


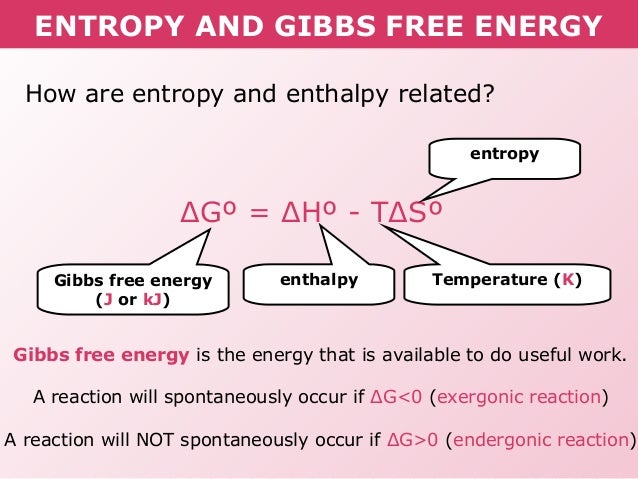
/chapter13/pages3and4/page3and4_files/gibbs_energy.png)