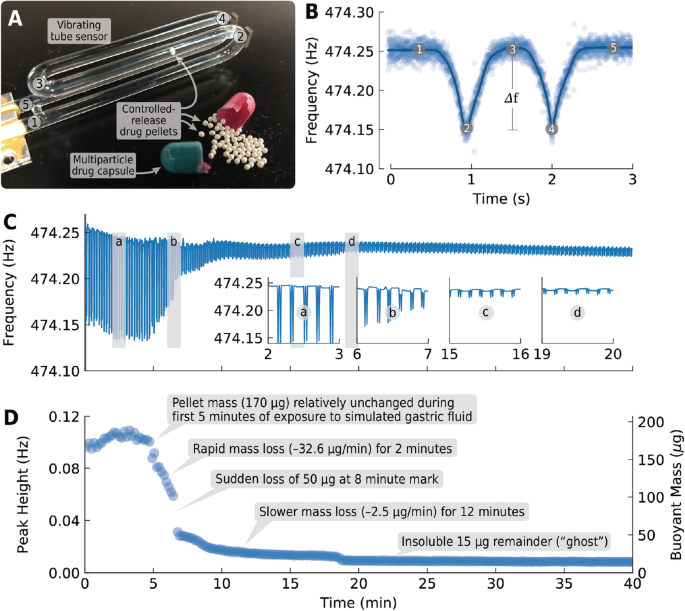
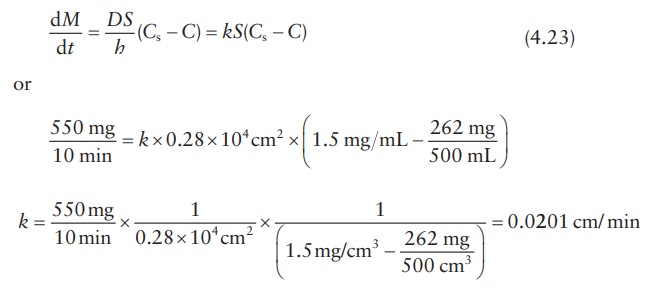
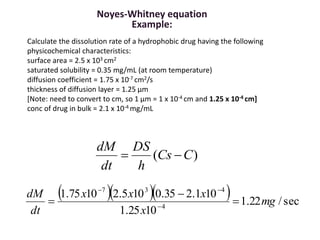
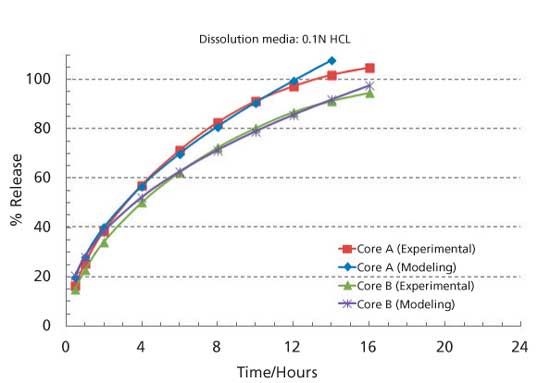
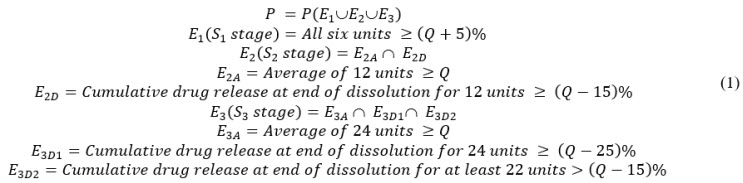A New Method for Evaluating Actual Drug Release Kinetics of Nanoparticles inside Dialysis Devices via Numerical Deconvolution - ScienceDirect
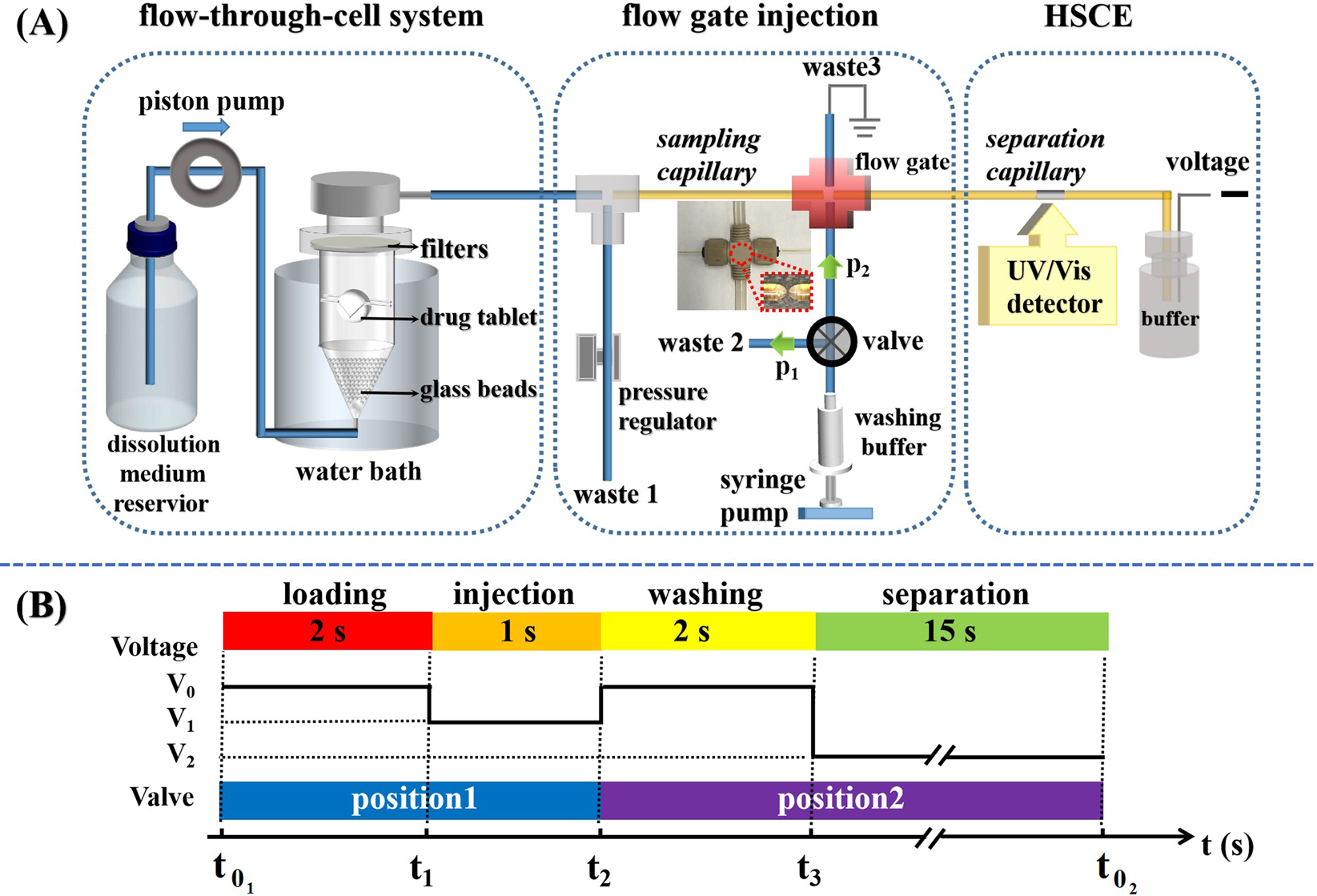
Automatic Dissolution Testing with High-Temporal Resolution for Both Immediate-Release and Fixed-Combination Drug Tablets | Scientific Reports

Real-time release testing of dissolution based on surrogate models developed by machine learning algorithms using NIR spectra, compression force and particle size distribution as input data - ScienceDirect
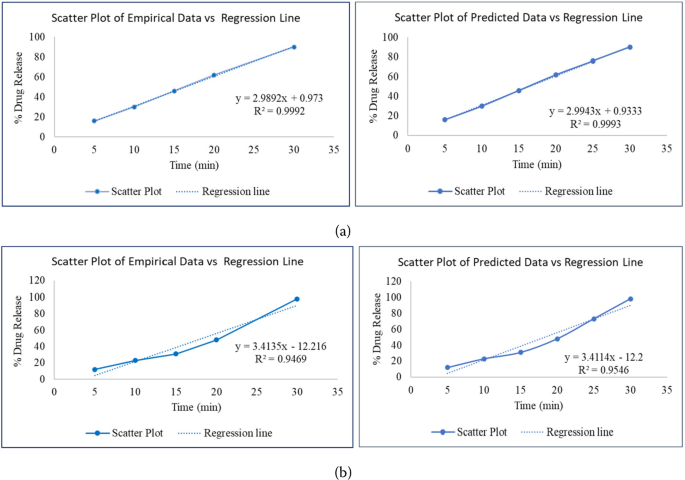
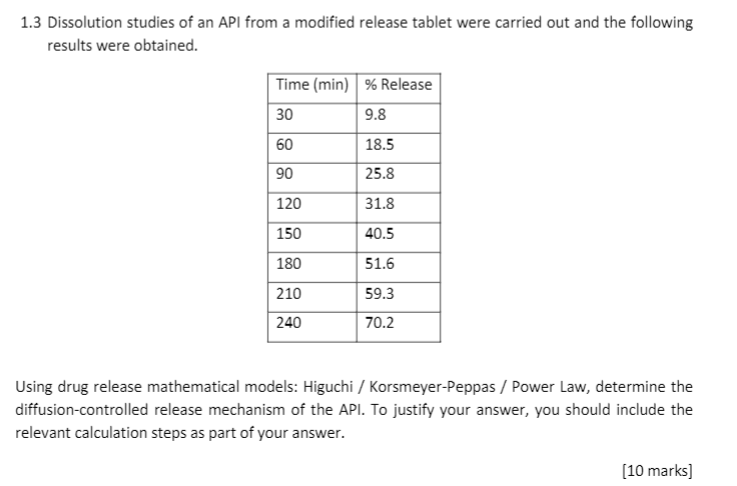
Optimizing similarity factor of in vitro drug release profile for development of early stage formulation of drug using linear regression model | Journal of Mathematics in Industry | Full Text

Disintegration, In vitro Dissolution, and Drug Release Kinetics Profiles of k-Carrageenan-based Nutraceutical Hard-shell Capsules Containing Salicylamide

In Vitro–In Vivo Correlation in Cocrystal Dissolution: Consideration of Drug Release Profiles Based on Coformer Dissolution and Absorption Behavior | Molecular Pharmaceutics

A Simple One-Parameter Percent Dissolved Versus Time Dissolution Equation that Accommodates Sink and Non-sink Conditions via Drug Solubility and Dissolution Volume | SpringerLink

Biopharmacotechnical and physical properties of solid pharmaceutical forms containing rutin commercially acquired in Juiz de Fora city, Brazil
![PDF] In-Vitro Dissolution Study and Shelf Life Calculation of Developed Sol-To-Gel Ocular Drug Delivery System of Brimonidine for Conjunctivitis during Accelerated Stability Study | Semantic Scholar PDF] In-Vitro Dissolution Study and Shelf Life Calculation of Developed Sol-To-Gel Ocular Drug Delivery System of Brimonidine for Conjunctivitis during Accelerated Stability Study | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/095f28a3e1119f309d356c3494689d68e9a0cebf/3-Table5-1.png)
PDF] In-Vitro Dissolution Study and Shelf Life Calculation of Developed Sol-To-Gel Ocular Drug Delivery System of Brimonidine for Conjunctivitis during Accelerated Stability Study | Semantic Scholar